Structure of an Atom
Everything around us — from the air we breathe to the food we eat — is made up of atoms. Atoms are the smallest units of matter that retain the properties of an element. Although atoms are incredibly tiny, they have a complex internal structure made of even smaller particles.
What Is an Atom?
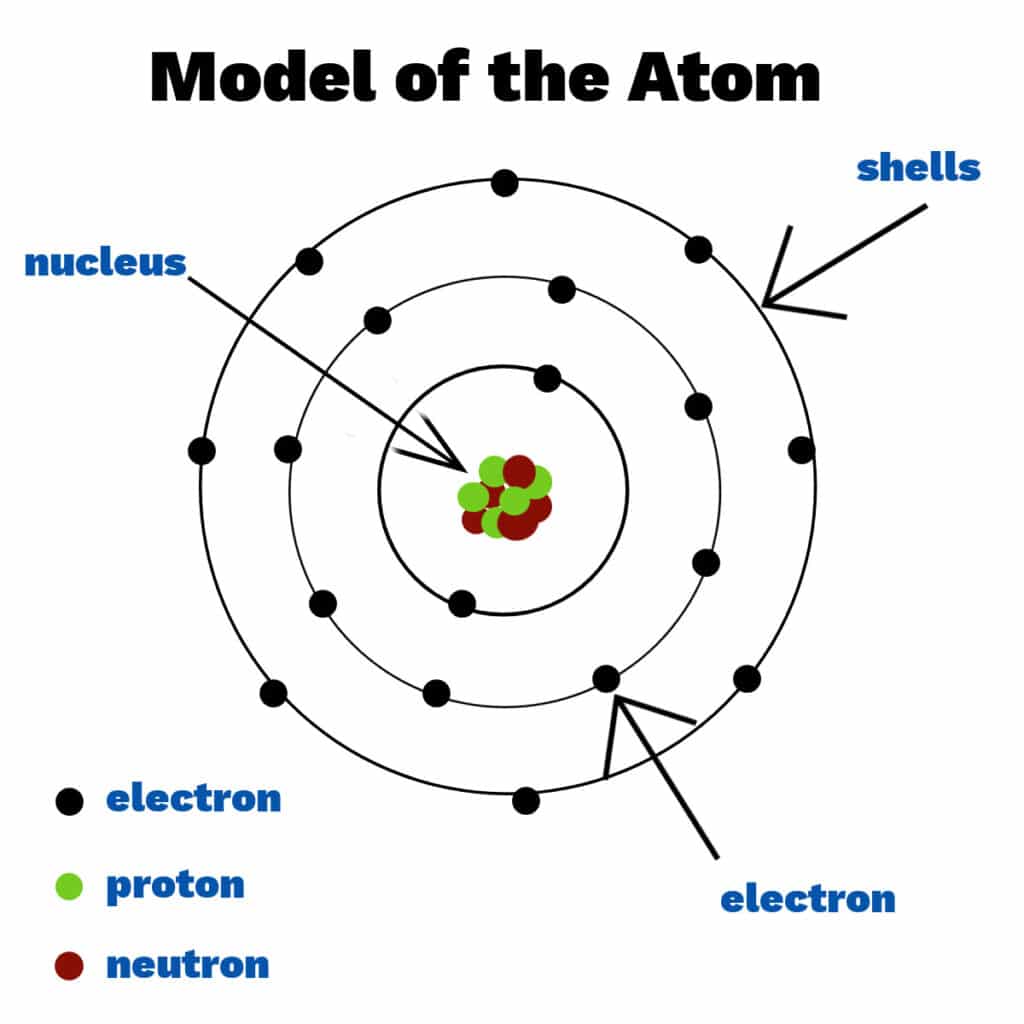
An atom is the basic building block of matter. Each atom consists of a central nucleus and a surrounding cloud of electrons. The type of atom (or element) is determined by the number of protons in its nucleus.
Parts of an Atom
- Protons: Positively charged particles found in the nucleus. The number of protons defines the element (e.g., hydrogen has 1 proton).
- Neutrons: Neutral particles with no charge, also located in the nucleus. Neutrons help stabilize the atom.
- Electrons: Negatively charged particles that orbit the nucleus in energy levels or shells. They are much smaller than protons or neutrons.
The Nucleus
The nucleus is the dense center of the atom. It contains protons and neutrons and holds nearly all of the atom’s mass. Despite its small size, the nucleus has a strong positive charge due to the protons.
Electron Shells and Orbitals
Electrons move around the nucleus in areas called shells or energy levels. Each shell can hold a certain number of electrons. The arrangement of electrons affects how atoms interact with each other and form chemical bonds.
- 1st shell: Up to 2 electrons
- 2nd shell: Up to 8 electrons
- 3rd shell: Up to 18 electrons
Atomic Number and Mass Number
- Atomic Number: The number of protons in an atom. This number is unique for each element.
- Mass Number: The total number of protons and neutrons in the nucleus.
Isotopes
Atoms of the same element with different numbers of neutrons are called isotopes. For example, carbon-12 and carbon-14 are both forms of carbon, but they have different mass numbers.
Why Atom Structure Matters
The structure of atoms determines how elements behave and react in nature. Understanding atoms helps explain everything from chemical reactions to electricity to nuclear energy.
Fun Facts
- Atoms are mostly empty space — if the nucleus were a golf ball, the electrons would be orbiting over a football field away.
- There are over 100 known elements, each made of unique atoms.
- The word “atom” comes from the Greek word "atomos," meaning "indivisible."
Conclusion: Atoms are the tiny yet powerful building blocks of the universe. By understanding the structure of atoms — protons, neutrons, and electrons — we can unlock the secrets of chemistry, physics, and how matter behaves.