The States of Matter
Matter is anything that has mass and takes up space. Everything around us — the air we breathe, the water we drink, the food we eat — is made of matter. Matter exists in different forms called states of matter. The four main states are solid, liquid, gas, and plasma. Each state has unique characteristics based on how the particles (atoms or molecules) inside are arranged and how they move.
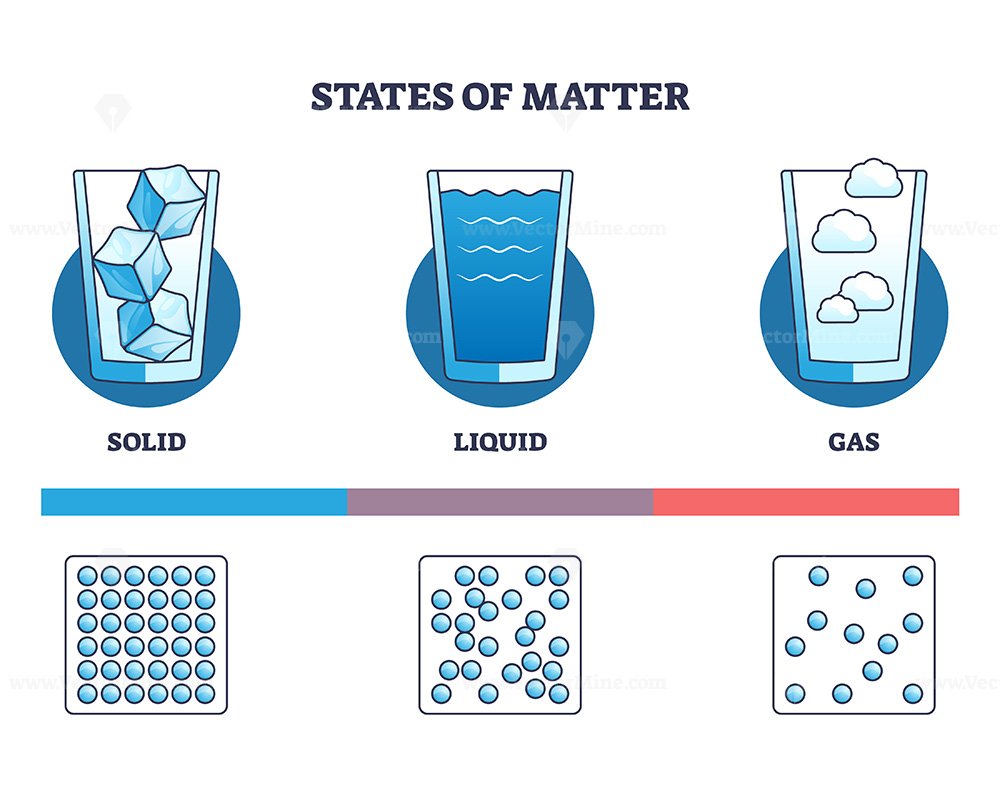
1. Solids
In a solid, the particles are packed closely together in a fixed arrangement. They vibrate in place but do not move freely. Solids have a definite shape and a definite volume. Examples of solids include ice, wood, metal, and rocks.
2. Liquids
In a liquid, the particles are still close together, but they can move past one another. This allows liquids to flow and take the shape of their container. Liquids have a definite volume but no definite shape. Common liquids include water, oil, and milk.
3. Gases
In gases, the particles are far apart and move freely in all directions. Gases have no fixed shape and no fixed volume — they expand to fill whatever space they are in. Examples include air, carbon dioxide, and helium.
4. Plasma
Plasma is a state of matter that is similar to gas but with one major difference: its particles are charged. This means they have lost or gained electrons. Plasma is found in stars, including the sun, and in lightning. It is the most common state of matter in the universe, even though it's not often seen on Earth.
Changing States of Matter
Matter can change from one state to another when energy is added or removed. These changes are called phase changes. Here are some examples:
- Melting: Solid to liquid (e.g., ice melting into water)
- Freezing: Liquid to solid (e.g., water freezing into ice)
- Evaporation: Liquid to gas (e.g., water turning into steam)
- Condensation: Gas to liquid (e.g., water vapor forming dew)
- Sublimation: Solid to gas without becoming a liquid (e.g., dry ice)
- Deposition: Gas to solid (e.g., frost forming on windows)
Why It Matters
Understanding states of matter helps explain how materials behave in different environments. From weather patterns to cooking to industrial processes, phase changes and properties of matter affect our daily lives.
Quick Facts
- Temperature and pressure affect the state of matter.
- Some substances can exist in more than one state at the same time.
- Plasma is used in neon lights and flat-screen TVs.
Conclusion: Matter is always in motion, and its state depends on how much energy the particles have. By learning about the four main states of matter and how they change, we can better understand the world around us — from the tiniest particle to the farthest star.